Spotlight: Ketamine’s Rapid Antidepressant Effects Mapped Brain-Wide + μ-opioid receptor?

Abstract:
Depression has been linked to cortico-limbic brain regions, and ketamine is known for its rapid antidepressant effects. However, how these brain regions encode depression collaboratively and how ketamine regulates these regions to exert its prompt antidepressant effects through mesoscale brain-wide fluctuations remain elusive. In this study, we used a multidisciplinary approach, including multi-region in vivo recordings in mice, chronic social defeat stress (CSDS), and machine learning, to construct a Mesoscale Brain-Wide Fluctuation Analysis platform (MBFA-platform). This platform analyzes the mesoscale brain-wide fluctuations of multiple brain regions from the perspective of local field potential oscillations and network dynamics. The decoder results demonstrate that our MBFA platform can accurately classify the Control/CSDS and ketamine/saline-treated groups based on neural oscillation and network activities among the eight brain regions. We found that multiple-region LFPs patterns are disrupted in CSDS-induced social avoidance, with the basolateral amygdala playing a key role. Ketamine primarily exerts the compensatory effects through network dynamics, contributing to its rapid antidepressant effect. These findings highlight the MBFA platform as an interdisciplinary tool for revealing mesoscale brain-wide fluctuations underlying complex emotional pathologies, providing insights into the etiology of psychiatry. Furthermore, the platform’s evaluation capabilities present a novel approach for psychiatric therapeutic interventions.
Cao, Q., Xu, X., Wang, X. et al. Mesoscale brain-wide fluctuation analysis: revealing ketamine’s rapid antidepressant across multiple brain regions. Transl Psychiatry 15, 155 (2025). https://doi.org/10.1038/s41398-025-03375-7
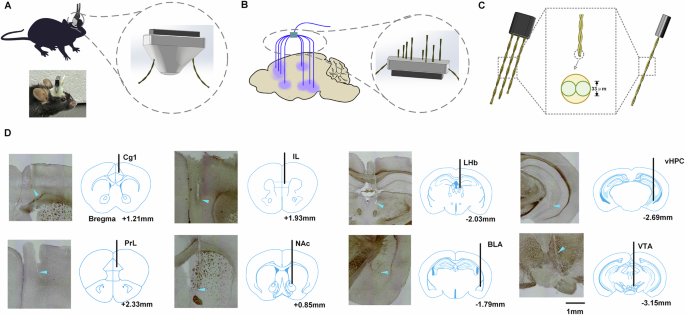

Pomrenze, M. B., Vaillancourt, S., Llorach, P., Rijsketic, D. R., Casey, A. B., Gregory, N., Zhao, W., Girard, T. E., Mattox, K. T., Salgado, J. S., Malenka, R. C., & Heifets, B. D. (2025). Ketamine evokes acute behavioral effects via μ-opioid receptor expressing neurons of the central amygdala. Biological psychiatry, S0006-3223(25)01177-1. Advance online publication. https://doi.org/10.1016/j.biopsych.2025.04.020
Focus of the Studies
- Study 1 (Opioid Receptor & Central Amygdala Study):
Focuses on the role of μ-opioid receptors (MORs) in the central amygdala (CeA) and their interaction with ketamine-induced hyperlocomotion in mice. It indirectly informs the antidepressant mechanism by suggesting that opioid signaling is involved. - Study 2 (Mesoscale Fluctuation Analysis):
Uses advanced imaging and signal processing to capture brain-wide network dynamics post-ketamine, showing how multiple regions (e.g., prefrontal cortex, hippocampus, thalamus) synchronize and reorganize to support rapid antidepressant effects.
Key Difference: Study 2 zooms in on a specific molecular and regional pathway (MOR in CeA); Study 1 zooms out to map global brain network dynamics.
Mechanism of Action
- Study 2:
Suggests opioid receptor signaling in the CeA is critical for some of ketamine’s acute behavioral effects, particularly hyperactivity, and potentially its antidepressant-like actions. - Study 1:
Proposes that ketamine induces widespread network reconfiguration, not limited to one region or receptor, with synchronized oscillations and increased neural flexibility (plasticity) being the key to symptom relief.
Implication: Study 2 supports the idea that specific neuromodulatory systems (opioids) are essential nodes in ketamine's effects, while Study 1 proposes a systems-level explanation rooted in network-wide dynamics and plasticity.
Methodological Approach
- Study 2:
Combines behavioral assays, cFos imaging, and region-specific pharmacology/genetics in mice. It's molecular and cellular in scale. - Study 1:
Employs mesoscale calcium imaging, electrophysiology, and machine learning for large-scale dynamic mapping in both animals and humans, offering real-time functional insights.
Method Strength: Study 1 provides temporal and spatial precision across whole-brain activity, while Study 2 provides causal evidence for a specific molecular mechanism.
Clinical Implications
- Study 2:
Warns that opioid receptor blockers like naltrexone may interfere with ketamine's therapeutic effects. It highlights a potential contraindication and a therapeutic target in MOR signaling. - Study 1:
Opens the door to personalized, network-targeted interventions (e.g., TMS, ultrasound), and better timing and dosing strategies by revealing how ketamine's effects evolve over time.
Clinical Relevance: Study 1 informs pharmacological caution and target identification; Study 2 enables precision psychiatry through system-level monitoring and prediction.
Compatibility or Conflict?
These two studies are complementary, not contradictory:
- Study 1 gives a mechanistic anchor—opioid signaling in the CeA is one of the gears in the ketamine machine.
- Study 2 shows how the whole machine runs—how ketamine reorganizes global brain function to produce therapeutic effects.
In essence, Study 2 could explain one mechanism feeding into the network shifts described in Study 1. The CeA and MORs might be critical in triggering the cascade that leads to the broader network plasticity revealed by the mesoscale analysis.
Conclusion
Together, these studies represent two critical layers of understanding:
- Molecular trigger (Study 2): Opioid signaling in emotion-processing regions like the CeA initiates ketamine’s effects.
- Network transformation (Study 1): These effects ripple through the brain, reconfiguring mood-related networks and enabling rapid antidepressant action.
This integrated view reinforces the idea that effective antidepressants may need to engage both local and global brain systems—restoring not just neurotransmitter levels, but also flexibility and communication across networks.