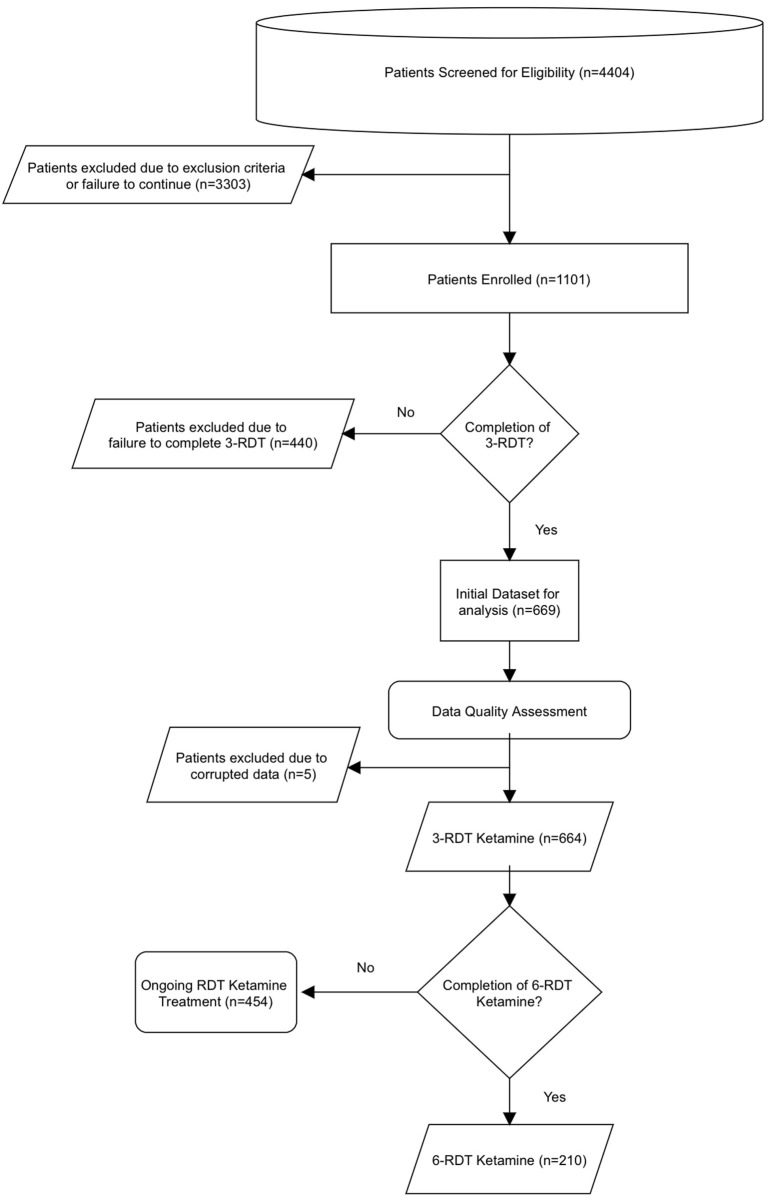
Sublingual & Oral Ketamine Research: Efficacy, Dosage, and Treatment for Mental Health

What does the research say about ketamine by mouth?
An ongoing review of the literature
Ketamine is a prescription medicine. Take only as directed by your medical provider.
There are various routes, formulations, and pharmaceutical companies studying ketamine and esketamine to bring to market in collaboration with the FDA in spite of racemic generic ketamine being a medicine prescribed by a medical clinician and formulated in local compounding pharmacies around the world since its FDA approval for anesthetic level dosing in humans and animals in 1970.
Ketamine is a medicine that has been studied non-stop since 1962. Ketamine may be prescribed with mental health support for a variety of indications as directed by a medical care provider. New routes of administration continue to be studied, including a new ketamine patch for pain, with the sponsor eying mental health indications for home use.

(2025) PharmaTher announcement for ketamine patch
It is important to keep this context mind in the discussion of appropriateness and safety concerns of medicines and routes of administration approved for home use, and the level of support offered. Clinical supervision with any prescription medicine is important. Medicines that may have psychological side effects are best with some form of human support. This includes ketamine assisted psychotherapy in an office, iv ketamine in a clinic, or ketamine at home through telemedicine to minimize misuse and harm. Like any psychedelic medicine, dosing and the experience you may have in a standard dosing range may be supported in extending the benefits beyond the acute effects of the medicine in the body and brain.
"A systematic review of PO (oral) and SL (sub-lingual) ketamine noted no significant side effects, and no problems with tolerance or substance misuse in any of the studies, which totaled 140 patients who received ketamine up to 3 years in duration…” (Swainson, 2022)

(2025): "Ketamine represents a significant advancement in antidepressant therapy, but the commonly used intravenous and intranasal application routes currently limit its availability beyond specialized centers. By contrast, oral ketamine treatment might constitute an alternative that is widely available, easy to administer, and has potential advantages with regard to tolerability."

(2025) Overall, these findings support the viability of voluntary oral ketamine administration and accentuate the need to refine the proposed model...
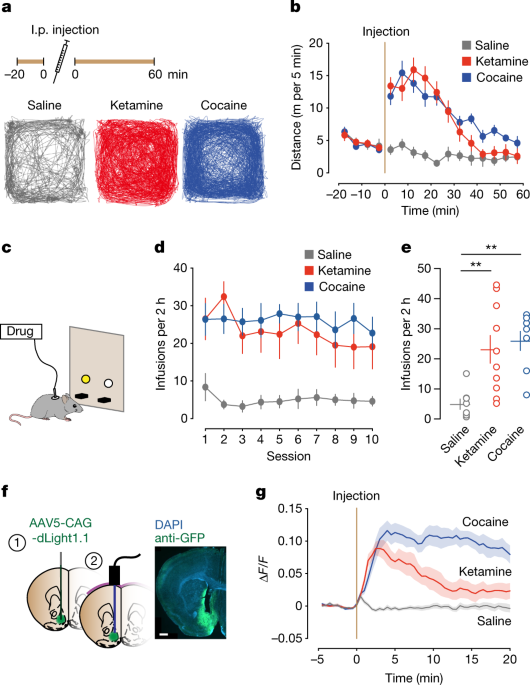
(2022): The rapid off-kinetics of the dopamine transients along with the NMDAR antagonism precluded the induction of synaptic plasticity in the VTA and the nucleus accumbens, and did not elicit locomotor sensitization or uncontrolled self-administration. In summary, the dual action of ketamine leads to a unique constellation of dopamine-driven positive reinforcement, but low addiction liability.

(2025) Ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist known for its rapid-acting antidepressant properties, has been extensively studied through intravenous and intraperitoneal routes. However, despite its growing use in pharmacotherapy, research on oral administration of ketamine remains limited. The antidepressant-like dose decreased the number of GluN2A and GluN2B expressing neurons in the paraventricular nucleus of the thalamus, basolateral amygdala, and habenula. High dose groups also showed diminished c-Fos+ cells in the medial habenula following acute stress. These results suggest that 45 mg/kg ketamine via oral gavage produces antidepressant-like effects, through regulation of NMDAR subunits in several depression-related structures like the medial habenula.

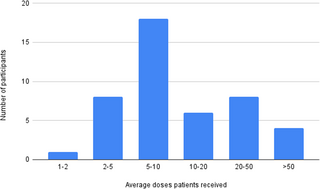
Ketamine for mental health - A naturalistic inventory of prescribing practices, safety, and adverse effects (Stuart, et al, 2025)



(2025) “Oral racemic ketamine is a reasonably well-tolerated and rapidly effective intervention for suicidal ideation and depression in adults with major depressive disorder.”

(2024) In TRD outpatients treated in general hospitals, oral ketamine may be better accepted and tolerated than iv ketamine.

(2024) The underlying brain mechanisms of ketamine in treating chronic suicidality and the characteristics of patients who will benefit from ketamine treatment remain unclear. To address these gaps, we investigated temporal variations of brain functional synchronisation in patients with suicidality treated with ketamine in a 6-week open-label oral ketamine trial. The trial's primary endpoint was the Beck Scale for Suicide Ideation (BSS). Patients who experienced greater than 50% improvement in BSS scores or had a BSS score less than 6 at the post-treatment and follow-up (10 weeks) visits were considered responders and persistent responders, respectively. The reoccurring and transient connectivity pattern (termed brain state) from 29 patients (45.6 years ± 14.5, 15 females) were investigated by dynamic functional connectivity analysis of resting-state functional MRI at the baseline, post-treatment, and follow-up. Post-treatment patients showed significantly more (FDR-Q = 0.03) transitions among whole brain states than at baseline. We also observed increased dwelling time (FDR-Q = 0.04) and frequency (FDR-Q = 0.04) of highly synchronised brain state at follow-up, which were significantly correlated with BSS scores (both FDR-Q = 0.008). At baseline, persistent responders had higher fractions (FDR-Q = 0.03, Cohen's d = 1.39) of a cognitive control network state with high connectivities than non-responders. These findings suggested that ketamine enhanced brain changes among different synchronisation patterns and enabled high synchronisation patterns in the long term, providing a possible biological pathway for its suicide-prevention effects. Moreover, differences in cognitive control states at baseline may be used for precise ketamine treatment planning.

(2024) About one-third of patients with depression do not achieve adequate response to current treatment options. Although intravenous and intranasal administrations of (es)ketamine have shown antidepressant properties, their accessibility and scalability are limited. We investigated the efficacy, safety, and tolerability of generic oral esketamine in patients with treatment-resistant depression (TRD) in a randomized placebo-controlled trial with open-label extension. This study consisted of 1) a six-week fixed low-dose treatment phase during which 111 participants received oral esketamine 30 mg or placebo three times a day; 2) a four-week wash-out phase; and 3) an optional six-week open-label individually titrated treatment phase during which participants received 0.5 to 3.0 mg/kg oral esketamine two times a week. The primary outcome measure was change in depressive symptom severity, assessed with the Hamilton Depression Rating Scale (HDRS17), from baseline to 6 weeks. Fixed low-dose oral esketamine when compared to placebo had no benefit on the HDRS17 total score (p = 0.626). Except for dizziness and sleep hallucinations scores, which were higher in the esketamine arm, we found no significant difference in safety and tolerability aspects. During the open-label individually titrated treatment phase, the mean HDRS17 score decreased from 21.0 (SD 5.09) to 15.1 (SD 7.27) (mean difference -6.0, 95% CI -7.71 to -4.29, p < 0.001). Our results suggest that fixed low-dose esketamine is not effective in TRD. In contrast, individually titrated higher doses of oral esketamine might have antidepressant properties.
This paper illustrates the importance of proper dosing if by mouth to achieve similar effects and safety profiles of other routes of administration of ketamine and its variant, esketamine. Similar outcomes were found in the last eleven years with racemic ketamine (Rolan, 2013, Nunez, 2020 , Swainson, 2020, Swainson, 2022, Hassan, 2022, Dutton, 2023).


(2024) A ketamine-containing tablet could be a convenient alternative to intravenous treatments, with fewer unpleasant side effects.

(2023) We posit that oral administration of ketamine for depression and suicidality similarly benefits from the norketamine effect observed in analgesia studies and further assert that oral administration of ketamine may be the route of choice, providing desirable clinical effects with less undesirable side effects associated with parenteral dosing. …emerging evidence suggests oral ketamine is a viable mental health treatment option; however, further research is required to provide a greater pharmacological understanding of oral ketamine, to inform dosing regimens for effective treatment and patient safety. Considering the current lack of evidence and consensus regarding a dosing protocol for oral ketamine, individually titrated dosing, within a risk versus benefit framework, is likely to be necessary to maximise both clinical response and safety and tolerability.

(2022) Given differences in kinetics, dosing, and bioavailability of PO (17%) [36], SL (29%) [37], and IN (50%) [38] ketamine formulations, it is unclear whether the safety and side effect profiles are entirely consistent across various formulations. A systematic review of PO and SL ketamine noted no significant side effects, and no problems with tolerance or substance misuse in any of the studies, which totaled 140 patients who received ketamine up to 3 years in duration… Side effects of IN ketamine have been more variable. One study found a dose of 50 mg was well tolerated [42], while another study of a series of 100-mg doses given over a period of weeks [43] was aborted early due to intolerable side effects of sedation and lack of motor coordination. In contrast, a small case series reported good tolerability of IN ketamine up to 150 mg administered up to twice weekly [31]. IN esketamine has a better documented safety profile, with common side effects of sedation, dissociation, and blood pressure changes that are noted to be mild and transient [6]. Hence, although evidence for PO, SL, and IN ketamine remains limited, no studies have reported concerns of cardiorespiratory compromise or clinically significant elevations in blood pressure that would preclude home use. It would seem logical that if the blood pressure effects of a 100% bioavailable IV formulation are rarely clinically significant, any blood pressure elevation from a less bioavailable non-parenteral formulation may be of variable duration, but not of greater magnitude, so long as approximately equivalent doses are given. No studies addressed the level of concern that would be warranted in regard to addictive potential of these non-parenteral formulations of ketamine, or potential differences in addictive potential given differing absorption and time to onset of dissociative effects. Given the lack of evidence and theoretical concern, it would be advisable to exercise similar caution with home use of ketamine as is given to the home use of stimulants or other sedatives. Indeed, the sedative GHB, a drug with significant side effects, overdose risk, cross tolerance with alcohol, abuse potential, and diversion potential [44], is indicated in a number of countries for narcolepsy with cataplexy and self-administered without medical supervision at home, under a controlled delivery program [44]."
(2022) …Oral ketamine, while less commonly used compared to the gold standard of IV ketamine, has been tested extensively from a pharmacodynamic and pharmacokinetic perspective. Specifically, oral ketamine has been shown to undergo extensive first-pass metabolism and consequently has approximately 10–20% bioavailability, while SL ketamine has a bioavailability of approximately 30% (42, 43), a fact which must be taken into account when designing outpatient oral or SL ketamine treatment regimens. A recently published manuscript has reported similar effectiveness and safety of at-home SL ketamine using a prospective study design (44). The authors found that SL ketamine (three treatments with doses ranging 300–450 mg) in combination with telehealth produced approximately 60% reduction in PHQ-9 and GAD scores at the 4-weeks timepoint compared to baseline scores, roughly comparable with our study. The improvements in depression and anxiety symptomology demonstrated in those who received as few as three at-home ketamine RDT present a safe, effective, and reasonable alternative to inpatient IV infusions of ketamine. These improvements in symptoms are further improved with completion of a clinically recommended course of six ketamine RDT. In addition to providing an effective treatment for those with TRD and comorbid anxiety, it also suggests alternative neurobiological modes for understanding MDD and anxiety symptoms. …Our findings indicate that SL ketamine is clinically effective in reducing depression and anxiety in an at-home setting. The use of SL ketamine at home presents a promising approach in the treatment of TRD where treatment resistance may be the result of prior treatment approaches being mismatched with underlying neurobiological etiology. Since most antidepressants target monoaminergic mechanisms, the effectiveness of ketamine's pharmacological profile suggests an underlying role for glutamate in some individuals who experience TRD (47).


(2020) SL ketamine can both provide and sustain rapid antidepressant effects. We suspect this difference may be a dose effect, and we wish to note that to determine appropriate dosing for future prospective studies, bioavailability of each formulation of ketamine must be considered. Sublingual ketamine is more bioavailable (30%) than oral ketamine (20%).2 A recent study3 described safety and efficacy of IV ketamine at doses of 0.5 mg/kg and 1.0 mg/kg and no benefit to lower doses. This translates to 1.5 or 3.0 mg/kg if dosed sublingually, and 2.5 or 5.0 mg/kg if dosed orally. With this in mind, only 2 (4,5) of 7 retrospective studies from the systematic review included patients with appropriately dosed ketamine. All others were below the equivalent expected SL and oral doses. Two prospective studies also underdosed ketamine at 25 mg bid (6) or or 0.5 mg/kg daily, (7) and 1 prospective study used a potentially adequate total daily dose of 50 mg tid, (8) but divided doses may have contributed to a reduced or slower ketamine response
(2020) Our quantitative analysis showed an antidepressant effect of oral ketamine that was significant and sustained from the 2nd week of treatment. Oral ketamine was well tolerated, although some studies lacked cardiovascular data such as blood pressure and heart rate. Our review did not show a significant increase in total side effects when we compared the individual psychiatric/gastrointestinal/neurological side-effects to placebo. Our results were in concordance with previous studies where authors highlighted a positive effect of oral ketamine.19,44

(2020) We hypothesize that the use of ketamine as an adjunctive treatment can have beneficial short-term and long-term effects on the course of BD. Previous studies, although still scarce have demonstrated the short-term antidepressant and antisuicidal effects of ketamine in patients with BD, and its administration also appears to carry a low risk of affective switch.7–36Studies on the molecular mechanism of action of ketamine indicate that it has effects on glutamatergic transmission, BDNF levels, and intracellular signal transduction, which are all perturbed in patients with BD. There is mounting evidence for the beneficial effect of ketamine on synaptogenesis and neuroplasticity; such long-term effects are particularly interesting, considering the neuroprogressive nature of BD.38–55,121 There is some evidence for the modifying effects of ketamine on epigenetic processes present in BD,64–70 as well as its ability to regulate inflammation.62–69 Ketamine may also have favorable effects on gut microbiota, which have been shown to be disturbed in patients with BD.71–78 The majority of the above-mentioned effects, however, are based on preliminary evidence and engage unipolar depression model, and therefore require confirmation by studies in bipolar disorder.




At home ketamine can be safe. Working with a licensed psychotherapist through a course of ketamine-assisted psychotherapy is one way to decrease risk.



Telehealth in psychotherapy - an introductory review of the literature
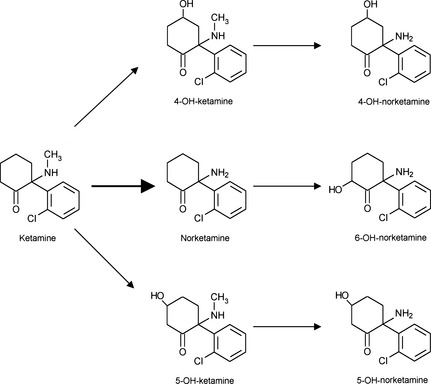
(2013)